GENEVA – “Game changer,” “a landmark decision for global health,” and “big times big plus big.” These are just a few of the reactions to the announcement from the European Commission (EC) that it has approved Merck’s Ervebo vaccine for the prevention of the Ebola virus.
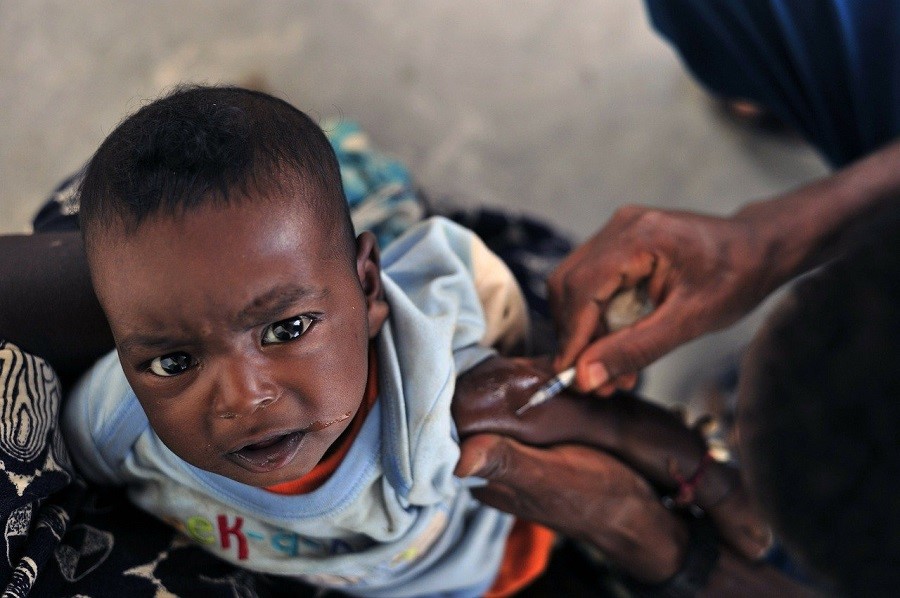
The vaccine has already been used in the Democratic Republic of Congo (DRC) under a “compassionate use” protocol to help stem the tide of the rapidly-spreading, deadly virus.
Nearly 2,200 people in the DRC have succumbed to the Ebola virus since its most recent outbreak in August 2018, making it the second-largest Ebola epidemic in history. On the upside, records indicate that the Ervebo vaccine has already contributed to the protection of more than 250,000 people who may have been exposed to the virus.
Merck’s CEO, Kenneth Frazier, hailed the approval as an
“historic milestone and a testament to the power of science, innovation, and public-private partnership.”
The European Medicines Agency (EMA) recommended the vaccine for approval by the EC in mid-October. The decision to approve the vaccine in an incomparably short time frame is a witness to the urgency of dealing with the spread of the disease and a testimony to the 97 percent efficacy rate. One member of the European Commission observed that
“Finding a vaccine as soon as possible against this terrible virus has been a priority for the international community ever since Ebola hit West Africa five years ago. Today’s decision is, therefore, a major step forward in saving lives in Africa and beyond.”
As noted, work on the vaccine became a global priority after the 2014 Ebola outbreak in which 11,000 people died. However, Canada’s National Microbiology Laboratory (CNML) had begun R & D on a vaccine not long after the Ebola virus had been recognized and isolated in 1976. Over the intervening decades, CNML research innovated several developments that ultimately made the production of an efficacious vaccine possible.
The vaccine has been licensed to Merck, which has the means of stockpiling and distributing it around the world as needed.
Studies still need to be conducted to determine how long the vaccine is effective in the human body.
Another item that needs attention is the development of vaccines for all four Ebola strains. Ervebo protects against the predominantly pervasive Zaire strain. According to the World Health Organization, seven other pharmaceutical companies are at various stages of clinical testing in their search for a vaccine.
Unlike most pharmaceuticals, neither Merck nor anyone else is likely to make a windfall from an Ebola vaccine. The virus has rarely been identified outside of areas in Africa where the residents are subsistence farmers who struggle to clothe, shelter, and feed their own families. The cooperation of governments, pharmaceutical companies, and NGOs will continue to be the source of funding that makes the vaccine available to all potential victims.
To read more news about the Ebola virus on Missions Box, go here.
Sources:
- Voice of America, EU Approval of First Ebola Vaccine is Game Changer
- Ars Technica, The world finally has an approved vaccine against Ebola
- Forbes, The Ebola Vaccine Is Now Officially Here With Approval In Europe
- Canadian Medical Association Journal, The story of Canada’s Ebola vaccine
- Nature, ‘Make Ebola a thing of the past’: first vaccine against deadly virus approved




